Zinc liberates hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not. Explain why?
Answer:
Zinc is above hydrogen whereas copper is below hydrogen in the activity series of metals. That is why zinc displaces hydrogen from dilute hydrochloric acid, while copper does not.
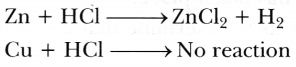
Zinc is able to displace Hydrogen from hydrochloric acid as it is more reactive than hydrogen while copper is less reactive than hydrogen hence, no reaction takes place.
zinc is more reactive than copper and hydrogen whereas copper is less reactive than hydrogen and zinc