Write the skeleton equations for the following chemical reactions and balance them by oxidation number method
1.Chloride ions reduce manganese dioxide to manganese (II) ions in acidic medium and get itself oxidised to chlorine gas.
2.Nitrate ions in acidic medium oxidise magnesium to ions but itself gets reduced to nitrogen (I) oxide.
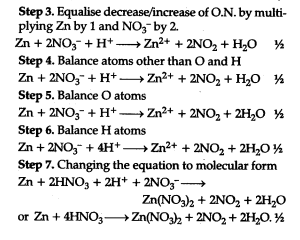
3.Zinc reacts with cone, nitric acid to produce zinc nitrate, nitrogen dioxide and water.