- Given that natural sample of iron has isotopes
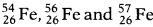 in the ratio 5%, 90% and 5% respectively. What will be the average atomic mass of Fe? Write any two applications of isotopes.
in the ratio 5%, 90% and 5% respectively. What will be the average atomic mass of Fe? Write any two applications of isotopes. -
- The relative atomic mass of boron is 10.8. Calculate the percentage of its isotopes
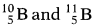 occurring in nature.
occurring in nature. - Write the number of neutrons present in C and C isotopes of carbon.
- The relative atomic mass of boron is 10.8. Calculate the percentage of its isotopes
- Average atomic mass of Fe =
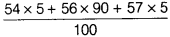 =
= 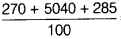 =
= 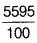 = 55.95 u
= 55.95 u
Two applications of isotopes are- C-14 is used to determine the age of old samples of fossils.
- Co-60 is used in the treatment of cancer (radiotherpy).
-
- Let the percentage of
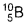 be Y in the sample and the percentage of
be Y in the sample and the percentage of 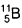 will be (100 - Y) .
will be (100 - Y) .
Average mass =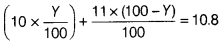
10Y + 11(100 - Y) = 1.08 x 100
10Y + 1100 - 11Y = 108
⇒ Y = 20
Therefore, the percentage of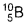 = 20% and percentage of
= 20% and percentage of 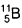 = 100 - 20 = 80%.
= 100 - 20 = 80%. - Number of neutron in
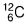 = Mass number - atomic number = 12 - 6
= Mass number - atomic number = 12 - 6
Number of neutrons in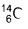 = 14 - 6 = 8
= 14 - 6 = 8
- Let the percentage of