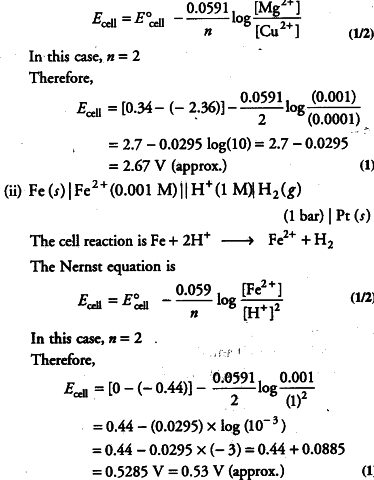
Write the Nernst equation and emf of the following cell at 298 K.
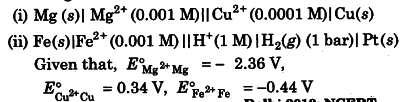
Write the Nernst equation and emf of the following cell at 298 K.
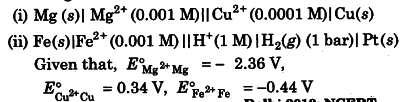
(i) For the given cell, the half-cell reactions will be given as below:
At anode Mg ------> {{Mg}^{2+}} + {{2e}^{-}}
At cathode {{Cu}^{2+}} + {{2e}^{-}} ------> Cu
Therefore, the overall cell reaction will be
Mg + {{Cu}^{2+}} -------> {{Mg}^{2+}} + Cu
The Nernst equation is