Write the names and symbols of two most reactive metals. Explain by drawing electronic structure how any one of them reacts with a halogen. Explain any two physical properties of the compound formed.
Answer:
Most reactive metals are Na (sodium) and K (potassium)
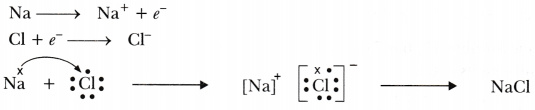
Physical properties:
- Physical nature: Hard and solid due to strong attractive forces between oppositely charged ions.
- High melting point and boiling point because more amount of energy is required to break strong force of attraction.