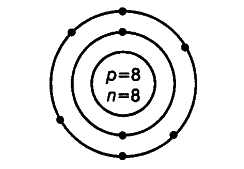
(i) Write the name of an element whose atom has same number of sub-atomic particles. Draw the atomic structure of the atom.
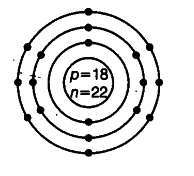
(ii) Draw atomic structure of an atom with same number of electrons in L and M-shells.
(i) Oxygen
Number of electrons = 8
Number of protons = 8
Number of neutrons = 8
(ii) Name of element: Argon
Atomic number = 18 = 2,8, 8
Number of electrons in L-shell = 8
Number of electrons in M-shell = 8