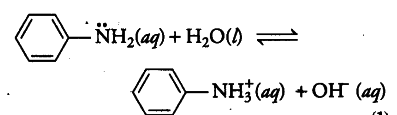(i) Write the equilibrium equation for the acid-base reaction that takes place between aniline and water.
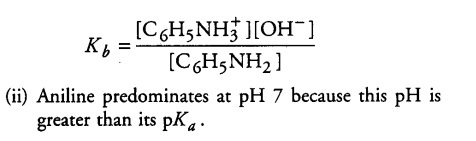
(ii) Being attached to an aromatic ring causes the —NH2 group of aniline to be less basic than non-aromatic amines, such as propylamine. Because aniline is a weak base, its conjugate acid is relatively r strong (pKa = 4.6). Which predominates at pH 7, aniline or its conjugate acid?
(i) An aqueous solution of a weak base in a state of equilibrium would consist mainly of the unionised form of the base, only a small amount of hydroxide ions and conjugate acid of the weak base is produced.