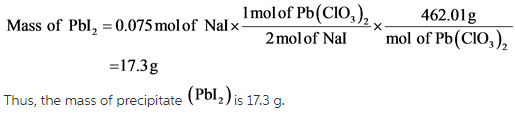Write the balanced equation for the reaction of aqueous Pb(ClO3)2 with aqueous NaI. Include phases.
What mass of precipitate will form if 1.50 L of concentrated Pb(ClO3)2 is mixed with 0.500 L of 0.150 M NaI? Assume the reaction goes to completion.
Concepts and reason
The mass of products produced from the reactants depends on the amount of limiting reactant and the moles of reactants and products present in a balanced chemical equation. Limiting reactant is the substance which is present in lesser amount than required to react with the other reactant.
Fundamentals
The balanced chemical equation is the equation in which the numbers of atoms of different elements present on the left hand side of the equation is equal to that of in right hand side.
Answer:
Lead (II) chlorate reacts with sodium iodide to form lead (II) iodide and sodium chlorate.
The reaction is as follows:
![]()
The balanced equation is as follows:
![]()
![]()
The lead atoms are equal on both sides of the reaction. To balance the sodium atoms, put the coefficient to for the reactant NaI. Now, all the atoms of each element are balanced on both sides of the reaction.
According to the balanced chemical equation, 1 mole of ![]() reacts with 2 moles of Nal.
reacts with 2 moles of Nal.
It is given that 1.50 L of concentrated ![]() is mixed with 0.500 L of 0.150 is mixed with 0.500 L of 0.150 M NaI.
is mixed with 0.500 L of 0.150 is mixed with 0.500 L of 0.150 M NaI.
Here, limiting reactant is NaI.
Calculate the number of moles of NaI as follows:
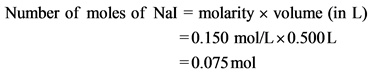
Now, calculate the number of moles of ![]() as follows:
as follows: