Write drawbacks of Rutherford’s atomic model.
Drawbacks of Rutherford’s Model of an Atom
-
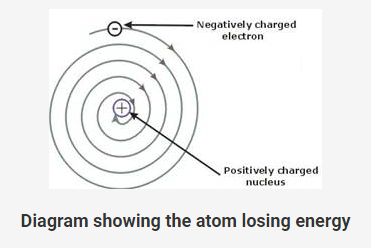
Rutherford’s atomic model could not explain how the moving electrons could remain in its orbit.
-
Any charged particle during acceleration would give out energy; and while revolving it would lose energy and eventually fall into the nucleus.
-
This means that the atom would be highly unstable.
-
But, matter is composed of stable atoms.
-
Thus, the major drawback of Rutherford’s atomic model was that it could not explain the stability of atoms.