Aim : To show the reaction of acids with metals.
Required Materials : 1. Test tube 2. Delivery tube 3. Glass trough 4. candle 5. Soap water 6. Dil. HCl 7. Zinc granules.
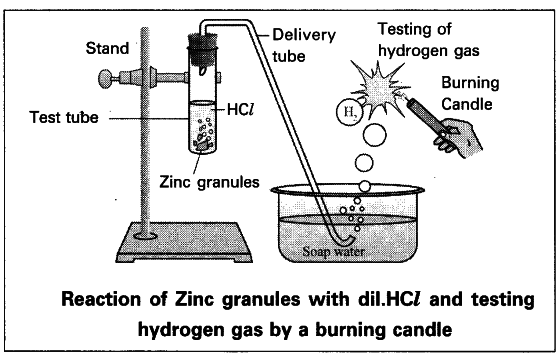
Procedure :
- Set the apparatus as shown in figure.
- Take about 10 ml of dilute HCl in a test tube and add a few zinc granules to it.
- We will observe the formation of gas bubbles on the surface of zinc granules.
- Pass the gas being evolved through the soap water.
- Gas filled bubbles are formed in the soap solution which rise into the air.
- Bring a burning candle near the gas filled bubbles.
- The gas present in a soap bubble burns with a ‘pop’ sound.
Result: - Only ‘hydrogen’ gas burns making a ‘pop’ sound.
- So we will notice that gas evolved is { H }_{ 2 }.
Additional Experiment: - Repeat the above experiment with H_{2}SO_{4} and HN{{O}_{3}}.
- We observe the same observation of the HCl experiment.
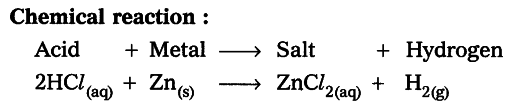
Conclusion : From the above activities we can conclude that when acid reacts with metal, ${ H }_{ 2 }$gas is evolved.