Write an activity to show that whether all compounds containing hydrogen are acids or not.
Procedure :
- Prepare glucose, alcohol, hydrochloric and sulphuric acid solution…
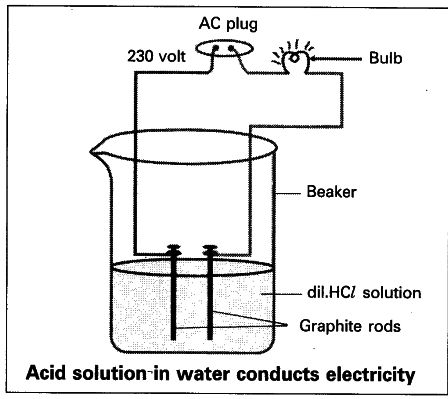
- Fix two iron graphite rods on a rubber cork and place the cork in a 100 ml beaker.
- Connect two different coloured electrical wires to graphite rods separately as shown in figure.
- Connect free ends of the wire to 230 volts AC plug.
- Complete the circuit as shown in the figure by connecting a bulb to one of the wires.
- Now pour some dilute HCZ in the beaker and switch on the current.
Observation : The bulb starts glowing.
Repeatation : Repeat activity with dilute sulphuric acid, glucose and alcohol so-lutions separately.
Observation : - We will notice that the bulb glows only in acid solutions.
- But the bulb not glows in glucose and alcohol solutions.
Result: - Glowing of bulb indicators that there is flow of electric current through the solution.
- Acid solutions have ions and the movement of these ions in solution helps for flow of electric current through the solution.
Conclusion : - The positive ion (cation) present in HCl solution is {{H}^{+}}.
- This suggests that acids produced hydrogen ions H+ in solution, which are responsible for their acidic properties.
- In glucose and alcohol solutions the bulb did not glow indicating the absence of {{H}^{+}} ions in these solutions.
- The acidity of acids is attributed to the {{H}^{+}} ions produced by them in solutions.