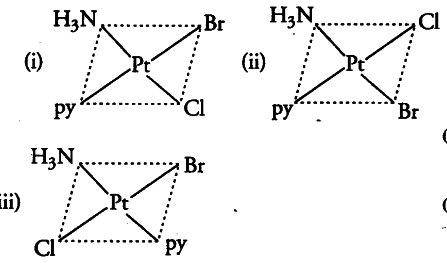
There are three geometrical isomers of (These structures are obtained by fixing the position of one ligand, i.e. NH _{3 } and placing at the trans-position any one of the remaining three ligands one by one. Two of these are cis and one is trans).
Optical isomerism is not exhibited by the compound with CN= 4 and square planar geometry because of the presence of horizontal plane of symmetry.