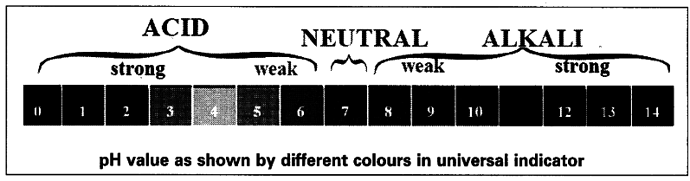
- A scale for measuring hydrogen ion concentration in a solution is called pH scale.
- pH value of a solution is simply a number which indicates the acidic or basic nature of a solution.
- If pH value = 7, it is a neutral solution
pH value < 7, it is an acidic.solution
pH value > 7, it is an basic solution - The range of pH value is from 0 to 14.
- As pH increases from 7 to 14, it represents a decrease in ${ H }_{ 3}$${{O}^{+}} ion concentration or an increase in {{OH}^{-}}$ ion concentration in the solution.