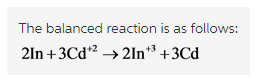Write a balanced overall reaction from these unbalanced half-reactions.
In---->In^3+
Cd^2+ ---->Cd
Concepts and reason
Write the given unbalanced reactions in two half reactions separately.
Balance the electrons. Add the two half reactions to get the final balanced reaction.
Fundamentals
A balanced equation is an equation which contains all the reactants and products in a fixed ratio. A balanced equation has the following characteristics:
- It should follow the law of conservation of mass.
- The total number of different types of elements on reactant side should be equal to the product side.
- Stoichiometric coefficients are put in front of the molecules on both the reactant and product side to balance a given reaction. The coefficients to be used are whole number or fractional number.
Answer:
The half reactions are written as follows:
![]()
On writing the electrons, the reactions obtained are as follows:
![]()
On balancing the electrons, the reactions obtained are as follows:
![]()
The balanced reaction is written as follows:
![]()