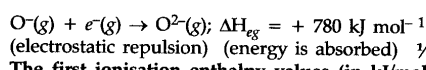When an electron is added to oxygen atom to form {{O}^{-}} ion energy is released. But when another electron is added to {{O}^{-}} ion to form {{O}^{2-}} ion energy is absorbed to overcome the strong electrostatic repulsion between the negatively charged {{O}^{-}} ion and the new electron being added. The second electron gain enthalpy of O is positive.
Thus, first electron gain enthalpy :
![]()
Second electron gain enthalpy :