With the help of a chemical equation, explain how a soda-acid fire extinguisher helps in putting out a fire.
Answer:
Soda-acid fire extinguisher contains sodium bicarbonate and sulphuric acid, which are in separate containers in them. When knob of the fire extinguisher is pressed, then sulphuric acid mixes with sodium bicarbonate solution and produces a lot ofC02 gas, which forms a blanket over the fire and cuts it off from the supply of the air to the burning substance and the fire stops.
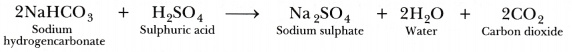
HCl + NaHCO3 --> NaCl + CO2 + H2O.
Can this reaction is used in above reaction