WHY TiCl3 is coloured whereas TiCl4 is colourless
The electronic configuration of Ti is [Ar]3d24s2 .
In TiCl3 the oxidation state of Ti is +3 while in TiCl4 the oxidation state of Ti is +4.
In case of TiCl3 there is one d electron present in 3d subshell. When light falls on the Ti3+ complex, the t2g electronis excited to eg level.this excitation take polace in greenish yellow region (≈ 5000 A°), the rest is transmitted. the complementary colour is transmitted which is violet. The colour due to this transition is called d-d transition.
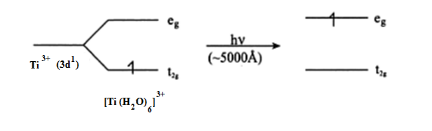
In the absence of ligands, crystal field splitting does not occur, colour disappears.Hence, anhydrous TiCl3 is colourless, but Ti(H2O)6Cl3 is violet.
In Ti+4 complex, there is no d electrons, hence d-d transition is not possible.