Why [Ti(H2O)6]3+ is violet in color but in [Ti(H2O)6]Cl3, when water molecules are removed it becomes colorless?
Co-ordination compounds exhibits color, which is attributed to the crystal field theory, corresponding to the d-d transition of elements. Say for example, excitation of an electron from the empty state say for example e.g., will absorb light that will excite the electron from t2g to eg as in case of [Ti(H2O)6]3+. When light corresponding to the energy of the yellow-green region is absorbed by the complex, this would excite the electron from t2g level to the eg level (t2g1eg0-> t2g0eg1). Thus the complex is violet in color.
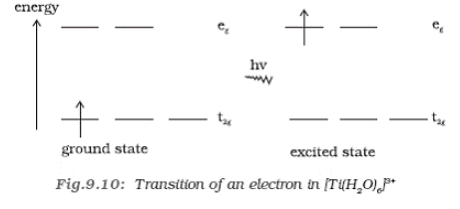
But in the absence of ligand there is no crystal field splitting and substance become colourless as in case of [Ti(H2O)6]Cl3, when water molecules are removed it becomes colourless.