Why lithium is strongest reducing agent?
Alkali metals are very good reducing agents because of their great tendency to lose electrons. The reducing character increases from Na to Cs .
However, Li is stronger reducing agent than Na due to greater hydration energy.
Explanation: An element, which acts as a reducing agent, must have low ionization energy. Alkali metals act as strong reducing agents as their ionization energy values are low. Since, ionization decreases on moving down from Li to Cs, the reducing property increases in same order. Thus, Li is the weakest reducing agent while Cs is the strongest reducing agent amongst alkali metals in free gaseous state .
The tendency of an element to lose electron in solution is measured by its standard oxidation potential value Eoext . Since, alkali metals have high Eoext values, these are strong reducing agents. However, it is observed that Li is the strongest reducing agent amongst alkali metals in solution as Eoext value of Li is maximum.
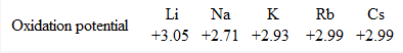
At first sight, lithium having high value of ionization energy amongst alaki metals acts as strongest reducing agent in solution. This can be explained if we understand the fact that ionization energy is the property of an isolated atom in gaseous state while oxidation potential is concerned when the metal atom goes into the solution.

Lithium being small in size has high ionization enthalpy. On the other hand because of small size it is extensively hydrated and has very high hydration enthalpy. This high hydration enthalpy compensates the high energy needed to remove electron (in second step). Thus Li has greater tendency to lose electrons in solution than other alkali metals. Thus, Li is the strongest reducing agent.