The oxidation state of N in nitrous acid (H—O—N=0) is +3 which lies in between its lowest oxidation state of —3 and highest oxidation state of +5. Since, the oxidation state of N in HN{{O}_{2}} can be decreased from +3 to any lower value, therefore, it acts an oxidising agent, e.g. HN02 oxidises H2S to S.
![]()
Further, since the oxidation state of N in HN{{O}_{2}} can be increased from +3 to +4 or +5, therefore, it acts as a reducing agent as well, e.g. HNOz reduces 12 and Br2 to HI and HBr respectively.
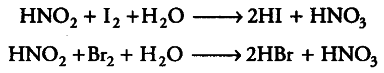
Thus, nitrous acid acts both as an oxidant as well as a reductant.