This is due to more resonance of benzylic free radical.
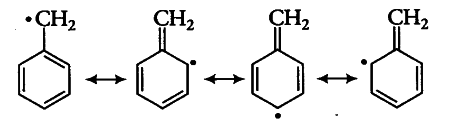
While allylic free radical has only two resonating structures soit has less delocalization.
![]()
This is due to more resonance of benzylic free radical.

While allylic free radical has only two resonating structures soit has less delocalization.
![]()
However, you are ignoring the fact that , allylic free radical too, can show 4 rsonating structures, because of hyperconjugation , AND , thermodynamically, too, ALLYL FREE RADICAL is MORE STABLE by 2kcal/mol .
This is due to the fact that Benzylic free radical has more no. of canonical structures as compared to Allylic free radical