Why is Aniline very reactive towards bromination?
Why is Aniline very reactive towards bromination?
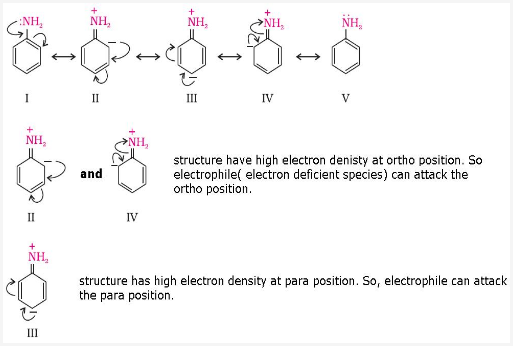
This is because NH2 group in aniline is highly activating group. A lone pair of electrons is present on N which releases the electron density to the benzene ring and hence activates the benzene ring towards electrophilic substitution reactions at ortho and para positions.
Since in bromination, Br+ is the electrophile. So, aniline reacts with bromine water at room temperature to give a white precipitate of 2,4,6 - tribromoaniline.