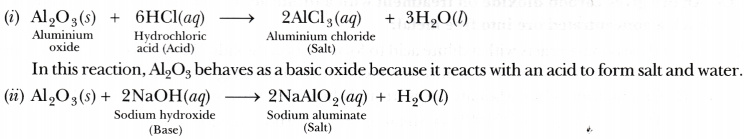Why is aluminium oxide considered an amphoteric oxide?
Answer:
Aluminium oxide (Al203) shows basic as well as acidic behaviour because it reacts with both acids and bases. Thus, it is considered an amphoteric oxide. The two types of reactions given by Al203 are as follows:
In this reaction, A1203 behaves as an acidic oxide because it reacts with a base to form salt and
water.