Consider the reactions,
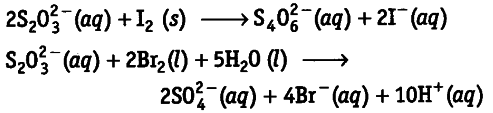
Why does the same reductant, thiosulphate react differently with iodine and bromine?
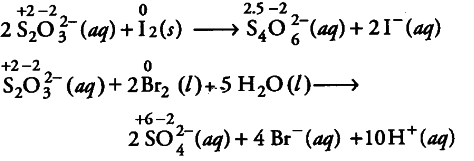
Bromine is a stronger oxidising agent in comparison to { I }_{ 2 }. It oxidises S of
$S_{2}O_{3}2- to a higher oxidation state + 6 in {{So}^{2-}}.
While { I }{ 2 }$ oxidises S of
$S{2}O_{3}$2-- to a lower oxidation state 2.5 in $S_{4}O_{6}$2–. That’s why same reductant, thiosulphate react differently with bromine and iodine.