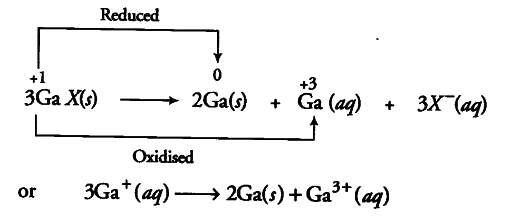
Gallium shows both +1 and +3 oxidation states due to inert pair effect. However, its +3 oxidation state is more stable than +1 oxidation state. Therefore, +1 gallium is less stable than +3 gallium and hence undergoes disproportionation to form gallium and more stable +3 gallium ions in aqueous solution as shown below .