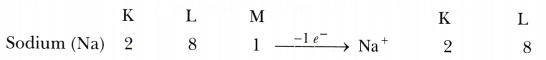Why do group 1 elements form unipositive ions?
Answer:
Group 1 elements contain 1 electron in their outermost shells. These elements lose this electron easily to attain the 8 electrons in their outermost shell. Hence, they form unipositive ion.
For example,