Which type of intermolecular forces exist between NaCl & ${{H}{2}}$O.
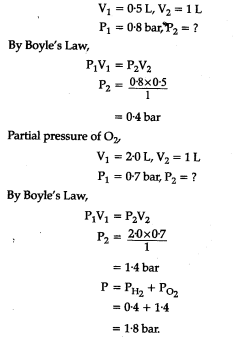
(b) What will be the pressure of the gas mixture when 0.5 L of ${{H}{2}}$ at 0.8 bar and 2.0 L of oxygen at 0.7 bar are introduced in a 1 L vessel at 27°C?
Ion - dipole interaction exists between an ion ({{Na}^{+}} or {{Cl}^{-}}) and a polar molecule (${{H}{2}}O). Thus, when NaCl is dissolved in water, the polar water molecules are attracted towards {{Na}^{+}} ion as well as {{Cl}^{-}} ion (hydration of ion).
(b) Partial pressure of {{H}{2}}$,