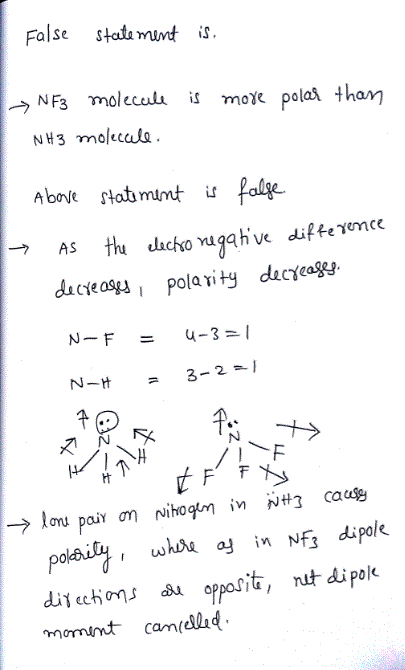
Which statement for NH3 and NF3 is false? Electronegativities N = 3.0, H = 2.1, F = 4.0.
-
The NF3 molecule is more polar than the NH3 molecule.
-
The bond angles in NF3 are smaller than those in NHS.
-
The bond dipoles in NF3 are directed toward the more electronegative fluorine atoms.
-
Both molecules have one unshared pair of electrons in the outer shell of nitrogen.
-
Both are sp3 hybridized at nitrogen.
-
The bond dipoles in NF3 oppose the effect of the unshared pair of electrons.
-
The nitrogen atom can be described as utilizing spJ hybrid orbitals in the nitrogen trifluoride molecule.
-
The bond dipoles of NF3 are directed toward fluorine, whereas those in NH: are directed toward nitrogen.
Answer: