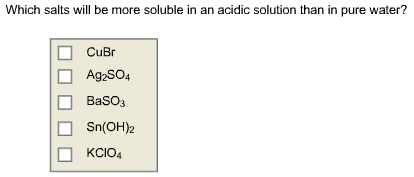
Which salts will be more soluble in an acidic solution than in pure water?
Answer:
the anion of a salt is able to react with a H+ ion to form a weak acid, it can be removed from the solution by adding an acid.
For AgI adding H+ forms HI and that is a strong acid; therefore, AgI is NOT more soluble in acid than in water.
For Sn(OH)2, adding H+ reacts with OH- to form H2O so YES, it is more soluble in acid than in H2O
For KClO4, adding H+ forms HClO4 but that is a strong acid so it is NOT more soluble in acid.
For CuBr adding H+ forms HBr and that is a weak acid; therefore, CuBr is more soluble in acid than in water.
For Ag2SO4 adding H+ forms HSO4- and that is k2 for H2SO4, there is no k1 since H2SO4 is a strong acid for the first H. so YES more soluble in acid than in water.
For BaSO3 adding H+ forms HSO3- and that is k2 for H2SO3 , there is no k1 since H2SO3 is a strong acid for the first H. so YES more soluble in acid than in water
SrSO4 would be HSO4^-
CaCO3 would be HCO3^- which is k2 for H2CO3 and YES.