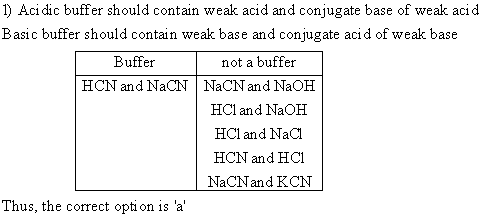
QUESTION 1
Which pair of compounds will form a buffer in aqueous solution?

QUESTION 2
A buffer solution is titrated with 0.032 M HCl solution. The initial volume of the buffer is 25.00 mL. The equivalence point of the titration is determined to be 4.88 mL. What is the concentration of conjugate base (in M) in the buffer solution? Assume the stoichiometry of acid to base is 1:1.
QUESTION 3
A buffer solution is titrated with 0.046 M NaOH solution. The initial volume of the buffer is 25.00 mL. The equivalence point of the titration is determined to be 8.77 mL. What is the concentration of acid (in M) in the buffer solution? Assume the stoichiometry of acid to base is 1:1.
QUESTION 4
The concentrations of acid and conjugate base in a buffer are determined experimentally to be 0.044 M and 0.059 M, respectively. If the total buffer concentration given on the bottle (the true value) is 0.102 M, then what is the percent error of the experimental value?
QUESTION 5
There are several calculations that need to be done in order to prepare a specific volume of buffer solution at a particular concentration and pH. There is a worked example in your lab manual on p. 119.
First, determine the correct A-/HA ratio: The pKa of acetic acid is 4.76, and the buffer solution needs to maintain a pH of 4.25. The concentration of acetate (CH3CO2-) should be X times that of the concentration of acetic acid (CH3CO2H). What is X?
QUESTION 6
There are several calculations that need to be done in order to prepare a specific volume of buffer solution at a particular concentration and pH. There is a worked example in your lab manual on p. 119.
Once the correct ratio of A- to HA is known, determine the actual concentration of A- needed to make a buffer at a particular concentration.
The ratio of acetate to acetic acid in a buffer is 0.176. That is, the concentration of acetate should be 0.176 times that of the concentration of acetic acid. What is the concentration of acetate (in M) needed to prepare a 0.416 M buffer using acetic acid and acetate?
QUESTION 7
There are several calculations that need to be done in order to prepare a specific volume of buffer solution at a particular concentration and pH. There is a worked example in your lab manual on p. 119.
What volume of acetate solution (in mL) is needed to make 250.0 mL of a buffer solution? The concentration of acetate in the buffer must be 0.0594 M, and the concentration of the acetate stock solution is 0.500 M.
Answer: