Which one of the following has the highest dipole moment?
(i) CH2$C{{l}{2}}$ (ii) chcl3
(iii) $CC{{l}{5}}$
The three dimensional structures of the three compounds along with the direction of dipole moment in each of their bonds are given below:
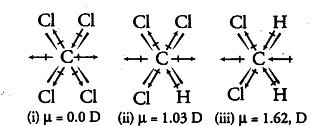
CCI4, being symmetrical, has zero dipole moment.
In CFIC13, the resultant of two C—Cl dipoles (1) is opposed by the resultant of C—H and C— Cl dipoles (2), since the latter resultant (2) is expected to be smaller than the former (1), so CHC13 must have finite dipole moment.