which of the following substances have polar interactions (dipole-dipole forces) between molecules?
F2, Cl2, ClF, NF3.
Concepts and reason
Dipole-dipole attractions: In polar molecules that have permanent dipoles, the dipoles are strongly attracted due to weak forces that are called as dipole-dipole attractions. These interactions are also called as polar interactions.
First identify the polar and nonpolar molecules.
Compare the electron negativity between the atoms and check the possibility of charge separations in the molecules. Basing on these points find out the possible interactions.
Fundamentals
A force by which atoms present in a molecule are attracted to one another through sharing of electrons is called as intramolecular force.
Polarity is attained to a molecule due to significant difference in electronegativity between the atoms in the molecule.
Answer:
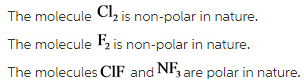
Explanation:

Explanation:

