Which is more polar : ${{CO}{2}}$ or
${{N}{2}}O$? Give reason.
${{N}_{2}}O$is more polar than
{{CO}_{2}}. This is because
{{CO}_{2}} is linear and symmetrical. Its net dipole moment is zero.
{{N}_{2}}O on the other hand, is linear but . unsymmetrical. It is considered as a resonance hybrid of the following two structures
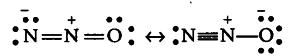
It has a net dipole moment of 0.116 D.