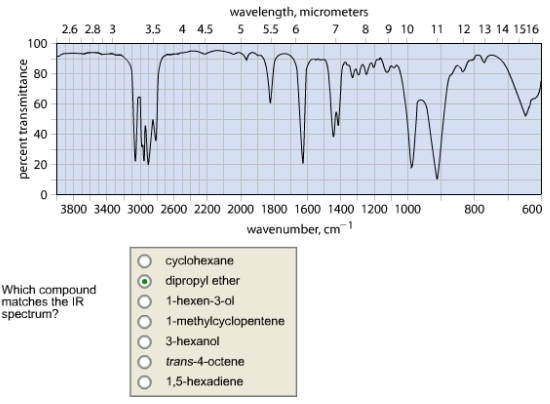
Which compound matches the IR spectrum?
Concepts and reason
Infra red spectroscopy identifies the structure and functional group of a compound. With the help of Infra red absorption range, the functional group of the structure and the double bond and triple bond that are present in the structure can be determined.
Fundamentals
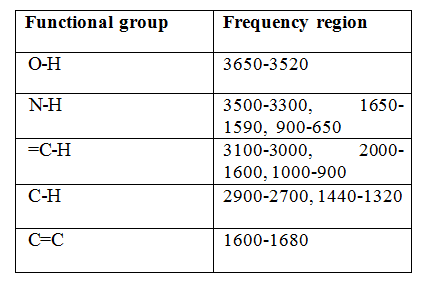
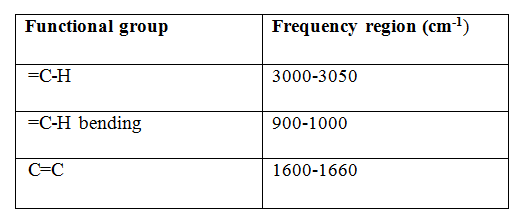
IR frequency range:
Answer:
Explantion:
There are 3 important stretches observed in the given spectrum. The![]()
![]() stretch is absorbed above 3000 cm-1. Then, the
stretch is absorbed above 3000 cm-1. Then, the ![]() out of plane bending of vinyl group was present above (900 cm-1), and the carbon – carbon double bond stretch occurs at 1600 – 1660 cm-1.
out of plane bending of vinyl group was present above (900 cm-1), and the carbon – carbon double bond stretch occurs at 1600 – 1660 cm-1.
Cyclohexane:
There are alkene peaks present in the spectrum. There are no double bonds present in cyclohexane. Therefore, it is not the correct structure.
Dipropyl ether:
![]()
There is no ether stretch present in the spectrum. Therefore, the above structure is not suitable for the given spectrum.
1 – Hexen – 3 – ol:
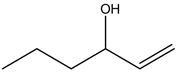
There is no alcohol stretch present in the spectrum. Therefore, the above structure is not suitable for the given spectrum.
1 – Methylcyclopentene:
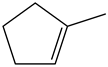
There is no vinyl bending present in the spectrum. Therefore, the above structure is not suitable for the given spectrum.
3 – Hexanol:
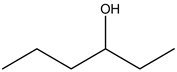
There is no alcohol stretch present in the spectrum. Therefore, the above structure is not suitable for the given spectrum.
Trans – 4 – octene:
![]()
There is no vinyl bending present in the spectrum. Therefore, the above structure is not suitable for the given spectrum.
1, 5 – hexadiene:
![]()
The vinyl bending and other stretches were matched with the above structure.
The IR spectrum matches with the compound.
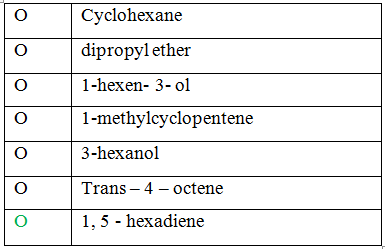
Explanation:
The c = c stretch, vinyl bending and ![]() stretch were matched with the above structure. Therefore, the given spectrum belongs to 1, 5 – hexadiene.
stretch were matched with the above structure. Therefore, the given spectrum belongs to 1, 5 – hexadiene.