(i) Which colour is imparted to flame by sodium?
(ii) Why are lithium salts commonly hydrated and those of the other alkali metal ions usually anhydrous?
(iii) Out of KOH and NaOH, which is a stronger base and why?
(i) Sodium imparts a golden yellow colour to the flame.
(ii) Because of its smallest size among alkali metals. Li+ has the maximum degree of hydration. That’s why lithium salts are commonly hydrated and those of other alkali metal ions usually anhydrous.
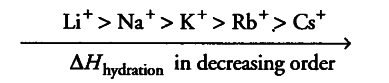
(iii) KOH is a stronger base than NaOH. Due to large size of K, K—O bond is weaker than Na—O bond. KOH has more concentration of OH than NaOH, hence, is a stronger base than NaOH.