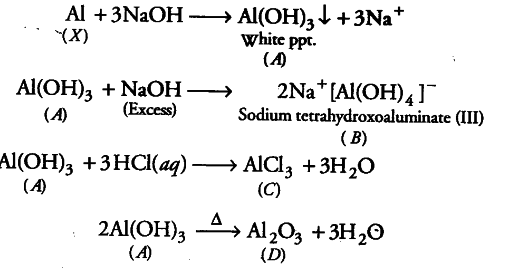
When metal X is treated with sodium hydroxide, a white precipitate (A) is obtained, which is
soluble in excess of NaOH to give soluble complex (B). Compound (A) is soluble in dilute HCl to form compound ©. The compound (4) when heated strongly gives (D), which is used to extract metal. Identify (X), (A), (B), © and (D). Write suitable equations to support their identities.
Since, mend X on treatment with sodium hydroxide gives white precipitate which dissolves in excess of NaOH to give soluble complex (B), therefore, the metal X is Al.