When aqueous solution of borax is acidified with hydrochloric acid, a white crystalline solid is formed which is soapy to touch. Is this solid acidic or basic in nature? Explain.
When an aqueous solution of borax is acidified with HCl, boric acid is formed.
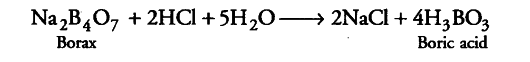
Boric acid is a white crystalline solid. It is soapy to touch because of its planar layered structure. Boric acid is a weak monobasic acid. It is not a protonic acid but acts as a Lewis acid by accepting electrons from a hydroxyl ion.
![]()