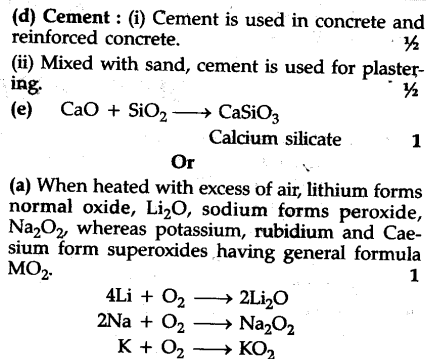
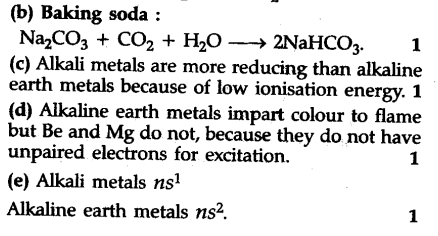(a) When alkali metals are heated in excess of air. What is the nature of oxides formed ?
(b) How can you prepare baking soda?
© Which is more reducing, alkali metals or ; alkaline earth metals ?
(d) Alkaline earth metals impart a characteristic colour to the flame but Be and Mg do not ; why ?
(e) Write general configuration of alkali and alkaline earth metals.