In the following table the mass numbers and the atomic numbers of certain elements are given.
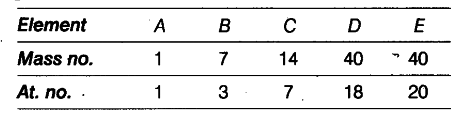
(i) Select a pair of isobars from the above table.
(ii) What would be the valency of element C listed in the above table?
(iii) Which two subatomic particles are equal in number in a neutral atom?
(i) D and E have same mass number but different atomic numbers. Hence, they are a pair of isobars.
(ii) Electronic configuration of C is 2(K), 5(L). Hence,
its valency is three because it gains three electrons to attain stable electronic configuration.
(iii) For a neutral atom,
Number of electrons = Number of protons
