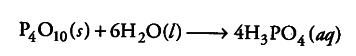What properties of water make it useful as a solvent? What type of compounds can it
(i) dissolve
(ii) hydrolyse?
High dipole moment and high dielectric constant, these are the two properties of water which make it useful as a solvent.
(i) It can dissolve both ionic compounds as well as those covalent compounds which can form hydrogen bonds with water such as ethyl alcohol, sugar, glucose, etc.
(ii) Water can hydrolyse many metallic and non-metallic oxides, hydrides, phosphides and other salts