What mass of ammonium chloride, NH4Cl, (Ka = 5.6 x 10^-10) must be added to exactly 500mL of 0.10M NH3 solution to give a solution with a pH of 9.00?
Concepts and reason
The concept used to solve this problem is based on the chemical equilibrium.
The pH of solution is used to determine acidity and basicity of a solution.
Fundamentals
The pH of solution can be determine by Henderson-Hasselbalch equation as follow.
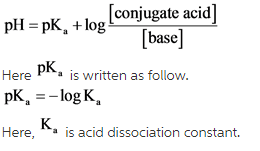
Answer:
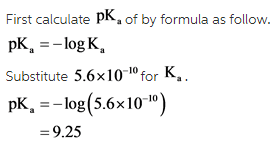
Write the balanced equilibrium reaction as follow.
![]()
Now, Henderson-Hasselbalch equation for this reaction is written as follow.
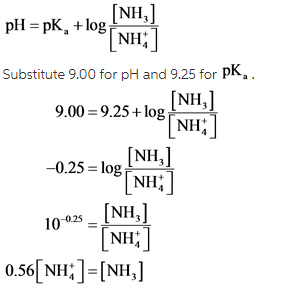
Explanation:
The Henderson-Hasselbalch equation is used to calculate pH of solution by using concentrations of base and its conjugate acid.