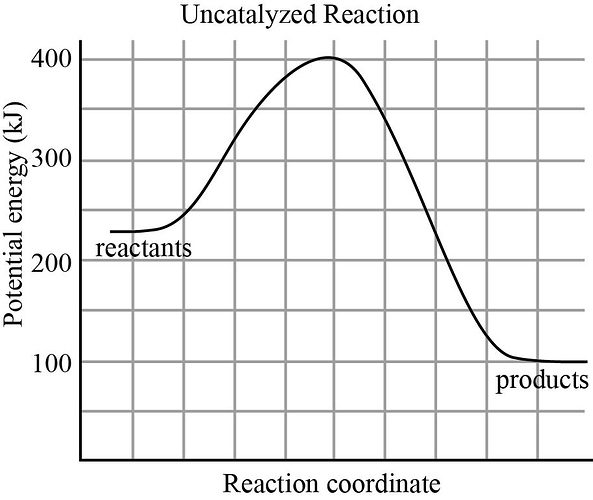
What is the value of the activation energy of the uncatalyzed reaction?
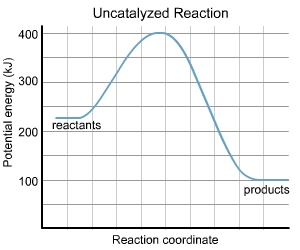
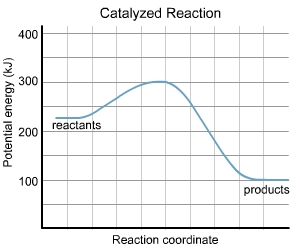
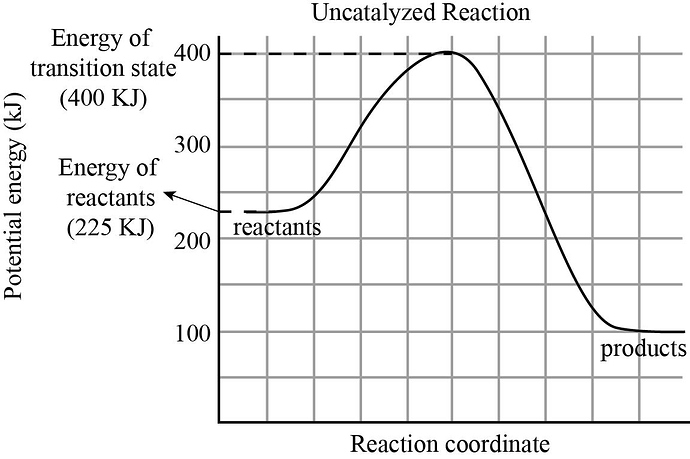
Please refer to the charts.
-
What is the value of the activation energy of the uncatalyzed reaction?
activation energy= -
What is the value of the enthalpy change of the uncatalyzed reaction?
delta H = -
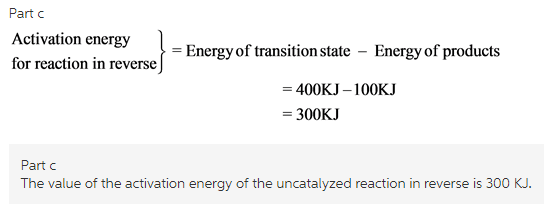
What is the value of the activation energy of the uncatalyzed reaction in reverse?
activation energy= -
What is the value of the enthalpy change of the uncatalyzed reaction in reverse?
delta H =
Concepts and reason
Catalyzed reaction involves a catalyst in the reaction. A catalyst just speeds up a chemical reaction but does not involve in the reaction mechanism. Uncatalyzed reaction does not involve catalyst in the reaction. Activation energy and enthalpy change for both catalyzed and uncatalyzed reaction can be found using the potential energy diagrams.
Fundamentals
A reaction that proceeds in a presence of a catalyst is known as catalyzed reaction. And a reaction that proceeds without a catalyst is known as uncatalyzed reaction.
At given same temperature and concentration of reactants, the activation energy of catalyzed reaction will be lower than the uncatalyzed reaction. And also catalyzed reaction needs less energy to reach the transition state than the uncatalyzed reaction.
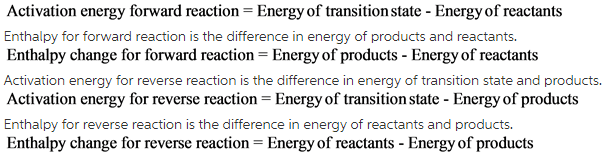
Formulae to calculate activation energy and enthalpy change for forward and reverse reaction is as follows:
Activation energy for forward reaction is the difference in energy of transition state and reactants.
Answer:
The potential energy diagram for uncatalyzed reaction is as follows:
The energy of transition state for uncatalyzed reaction is found to be 400KJ and energy of reactants for uncatalyzed reaction is found to be 225KJ.


Part d