- An element X has an atomic number = 12 and mass number = 26. Draw a diagram showing the distribution of electrons in the orbits and the nuclear composition of the neutral atom of the element. What is the valency of the element and why?
- If this element X combines with another element Y whose electronic configuration is 2,8,7. What will be the formula of the compound thus formed? State how did you arrive at this formula.
- Atomic number =12
Mass number =26
Atomic structure of X
Electronic configuration = 2, 8, 2
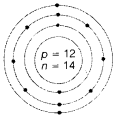
Nuclear composition
Number of protons = 12
Number of neutrons =26-12 = 14 (1)
Valency = 2 (1/2)
Because it can donate 2 electrons easily to complete its octet and become stable. - Valency of the element Y would be 1, as it can gain 1 electron to become stable. When it combines with the element X of valency 2, the compound formed will be XY2.
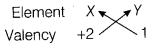
Formula of the compound would be XY2.