What is the state of hybridisation of carbon in
(i) ${ CO }_{ 3 }^{ 2- }$ (ii) diamond (in) graphite?
The state of hybridisation of carbon in { CO }_{ 3 }^{ 2- } , diamond and in graphite is {{sp}^{2}}, {{sp}^{3}} and {{sp}^{2}} respectively.
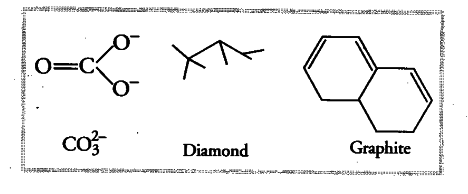
What is the state of hybridisation of carbon in
(i) ${ CO }_{ 3 }^{ 2- }$ (ii) diamond (in) graphite?
The state of hybridisation of carbon in { CO }_{ 3 }^{ 2- } , diamond and in graphite is {{sp}^{2}}, {{sp}^{3}} and {{sp}^{2}} respectively.
