What is the standard Gibbs free energy for the transformation of diamond to graphite at 298K?
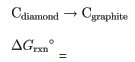
Elemental carbon usually exists in one of two forms: graphite or diamond. It is generally believed that diamonds last forever. Here are the standard enthalpy![]() of formation values for diamond and graphite.
of formation values for diamond and graphite.

Concepts and reason
The reaction is as follows:
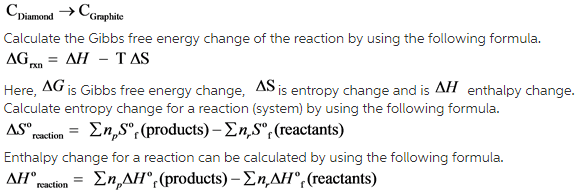
Fundamentals
Entropy is a thermodynamic quantity, it is a measure of randomness of a system.
Change in the enthalpy when one mole of a substance is formed from its pure elements under standard conditions is called as standard enthalpy of formation. The standard conditions are 1atm pressure and 298K temperature.
Answer:
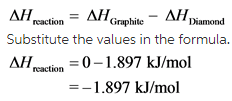

The value of enthalpy change of the reaction is calculated by substituting the given entropy values.

The value of free energy change of the transformation is calculated by substituting enthalpy change and entropy change of the reaction in the following formula.
