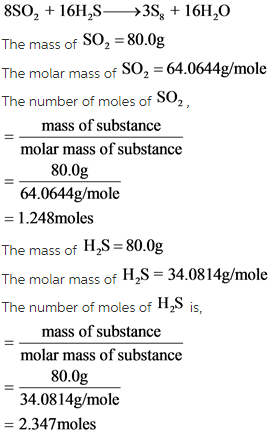
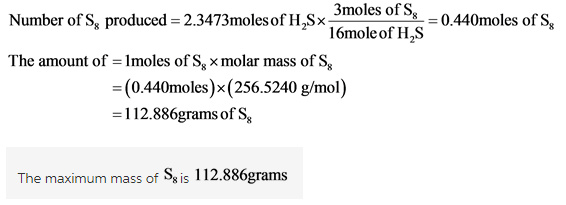
What is the maximum mass of S8 that can be produced by combining 80.0 g of each reactant?
8SO2+16H2S—>3S8+16H2O
= ? S8
Concepts and reason
Law of conservation of mass states that mass neither created nor destroyed in the chemical reaction. Mass of the substances can be calculated by using the balanced chemical equation and law of conservation mass.Theoretical yield of a reaction can be calculated by taking moles of limiting reactant in the reaction and their exact utilization
Fundamentals
Number of moles
Limiting reactant: Theoretically, the reaction takes place according to stoichiometric moles of reactants, if the reactants are not reacted according to the stoichiometric ratios, one of the reactants consumed completely after the completion of the reaction is limiting reactant and another reactant is in excess.
Theoretical yield:
In the reaction, Limiting reactant limits the amount of product formed, the moles of product formed in the reaction are calculated from the moles of limiting reactants available.
Answer:
The balanced chemical reaction is,
Explanation:
Moles of each reactant are calculated by identifying molar mass from the periodic table and dividing it by mass of each reactant.
For instance:
Calculate moles of X
From the balanced reaction, the molar ratio of reactants is 1:2which means the one mole of SO2 reacts with 2 moles of ![]() .
.
The stoichiometric ratio is,
Explanation:
Calculate the mass of the product.
Explanation:


