What is the IUPAC name for the compound shown below? The name should have the format alkanamine.
(1)
(2)
(3)
(4)
Concepts and reason
IUPAC stands for international union of pure and applied chemistry. IUPAC has given a nomenclature to name the organic compounds. The IUPAC name consists of three parts: root name, prefix and suffix.
Fundamentals
- The three parts of an IUPAC name are root name, prefix and suffix.
- The longest chain gives the root name.
- Prefix gives the position and name of the substitutions present on longest chain.
- Suffix gives the functional group present in the structure.
Answer:
(1)
The given structure of the compound is shown below.

Root name is ethan.
The longest chain has two carbon atoms. So, the root name for the given structure is ethan. Select the longest chain such that, the substituents have lowest numbers.
Make sure that the substituents on longest chain should be at lowest numbers.
Methyl group is attached to the nitrogen atom.

The prefix is N-methyl
One methyl group is attached to the nitrogen atom. So, the prefix is N-methyl.

The suffix is amine
Thus, the IUPAC name of the given structure is N-methylethanamine.
The functional group is amine. So, that the suffix is amine.
(2)
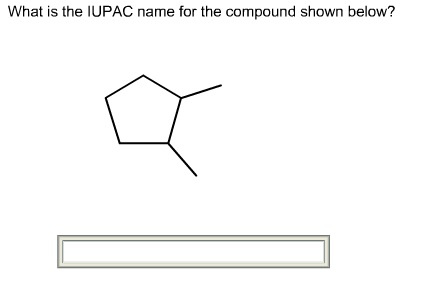
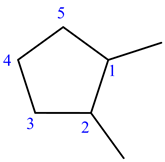
The given structure of the compound is shown below.
Root name is cyclopent.
The longest chain has five carbon atoms and it is in cyclic form. So, the root name for the given structure is cyclopent. Select the longest chain such that, the substituents have lowest numbers.
Two methyl groups are substituted at C-1 and C-2 carbons.

The prefix is 1,2-dimethyl
Two methyl groups are substituted at C-1 and C-2 carbons. So, the prefix is 1,2-dimethyl.

The suffix is ane
Thus, the IUPAC name of the given structure is 1,2-dimethylcyclopentane
The functional group is alkane. So, that the suffix is ane
(3)
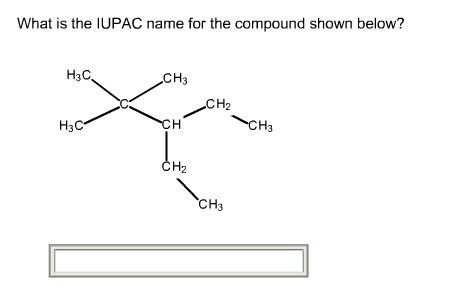
The given structure of the compound is shown below.
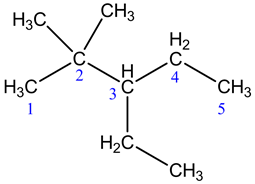
Root name is pent.
The longest chain has five carbon atoms. So, the root name for the given structure is pent. Select the longest chain such that, the substituents have lowest numbers.
Two methyl groups are substituted at C-2 carbons and one ethyl groups is substituted at C-3 carbon.
http://ec.images.prod.s3.amazonaws.com/8ee82c8ed9b04083a1a6cb0ba62f6364.png
The prefix is 3-ethyl-2,2-dimethyl
Two methyl groups are substituted at C-2 carbon and one ethyl groups is substituted at C-3 carbon. So, the prefix is 3-ethyl-2,2-dimethyl.
Make sure that the substituents are named in an alphabetical order.
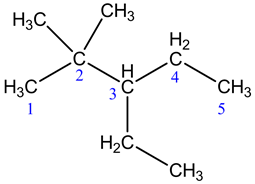
The suffix is ane
Thus, the IUPAC name of the given structure is 3-ethyl-2,2-dimethylpentane
The functional group is alkane. So, that the suffix is ane.
(4)
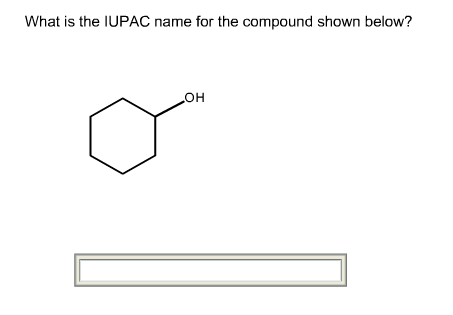
The given structure of the compound is shown below.
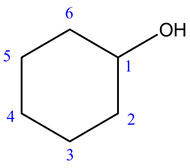
Root name is cyclohexan.
The longest chain has six carbon atoms and it is in cyclic form. So, the root name for the given structure is cyclohexan. Select the longest chain such that, the substituents have lowest numbers.
No substituents are present.

The prefix is absent.
No substituents are present. So, the prefix is absent.

The suffix is ol
Thus, the IUPAC name of the given structure is cyclohexanol.
The functional group is alcohol. So, that the suffix is ol.
- Thus, the IUPAC name of the given structure is N-methylethanamine
- Thus, the IUPAC name of the given structure is 1,2-dimethylcyclopentane
- Thus, the IUPAC name of the given structure is 3-ethyl-2,2-dimethylpentane
- Thus, the IUPAC name of the given structure is cyclohexanol
