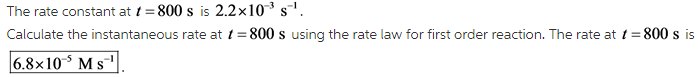
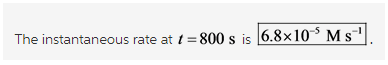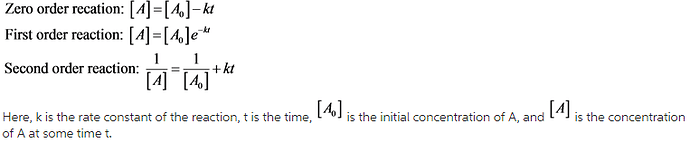What is the instantaneous rate of the reaction at t=800. s ? and the units please?
An average reaction rate is calculated as the change in the concentration of reactants or products over a period of time in the course of the reaction. An instantaneous reaction rate is the rate at a particular moment in the reaction and is usually determined graphically.
The reaction of compound forming compound was studied and the following data were collected
Time (s)
0. 0.184
200. 0.129
500. 0.069
800. 0.031
1200 0.019
1500 0.016
average reaction rate between 0 and 1500 is 1.12*10 to the negative fourth. M/s
average reaction rate between 500 and 1200s is 7.14*10 to the negative fifth.
Concepts and reason
This question is concerned with deriving the order of the reaction and the instantaneous rate at a particular time. This can done by plotting the change in concentration of the reactant with time.
Fundamentals
Consider the reaction given below:
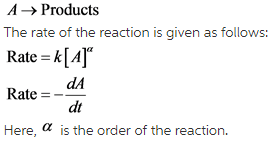
Depending on the rate of reaction different rate laws are obtained for the reaction. The rate law equation for a particular reaction gives the dependence of concentration change of the reactant with time.
Some of the rate laws are given below:
Answer:
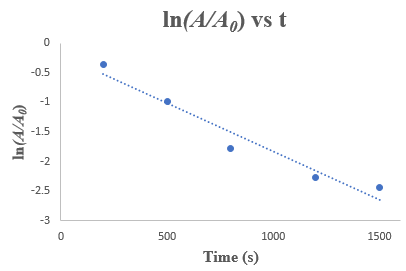
The reaction is a first order reaction because the concentration decreases exponentially with time.
Explanation:
Plot the given concentration of the reaction against time as follows:
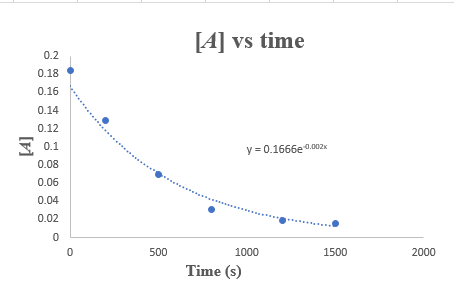
It can be seen from the graph that the concentration of the reactant decreases exponentially with time. Thus, this reaction is first-order.
The first order rate equation can also be written as follows:
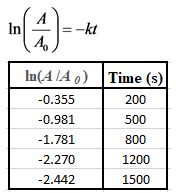
Plot a graph for this as shown below: