What is the hybridization, the number of lone pair electron and shape of XeOF2 ? Give reason.
XeOF2 has trigonal bipyramid geometry due to sp3d hybridisation.
Structure:
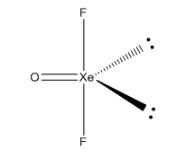
There are two lone pairs in the XeOF4, and it has T-shaped structure.
What is the hybridization, the number of lone pair electron and shape of XeOF2 ? Give reason.
XeOF2 has trigonal bipyramid geometry due to sp3d hybridisation.
Structure:

There are two lone pairs in the XeOF4, and it has T-shaped structure.