What is the gold foil experiment? Name the scientist who performed this experiment. Write the conclusions and shortcomings of Rutherford’s model of atom.
Answer:
In 1911, Rutherford performed the gold foil experiment. He bombarded a stream of alpha-particles on a gold foil, a thin sheet which was 0.00006 cm thick in an evacuated chamber. An a-particle is a positively charged helium ion (He2+). A simplified picture of this experiment is shown in the figure.
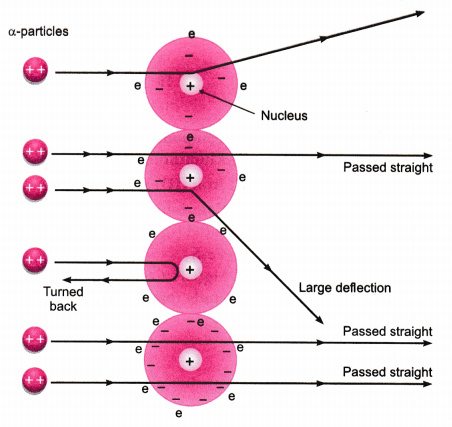
In this famous experiment, the following observations were made.
- Most of the a-particles passed straight through the foil without any deflection. This concluded that most of the space inside of an atom is empty.
- A few a-particles were deflected through small angle and few through larger angles. This happened due to positive charge on alpha-particles and core (nucleus) of the atom. The heavy positively charged ‘core’ was named as nucleus.
- The number of a-particles which bounced back was very small. This concluded that the volume of the nucleus is very small in comparison to the total volume of the atom.
On the basis of gold foil experiment, Rutherford concluded that an atom consists of nucleus which has positive charge and it is surrounded with electrons which are moving around the nucleus. The number of electrons and protons are equal and the entire mass of the atom is concentrated at its nucleus.
Drawbacks in the Rutherford’s model
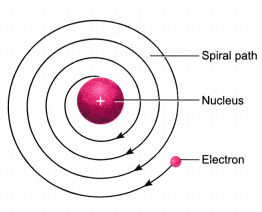
- According to classical electro-magnetic theory, a moving charged particle, such as an electron under the influence of attractive force loses energy continuously in the form of radiations. As a result of this, electron should lose energy and therefore, should move in even smaller orbits ultimately falling into the nucleus. But the collapse does not occur. There is no explanation for this behaviour.
- Rutherford did not specify the number of orbits and the number of electrons in each orbit.